Leiomyosarcoma of the Inferior Vena Cava
SectionCardiovascular
Case TypeClinical Cases
Authors
Mathias Abela1, Karl Buhagiar1, Malcolm Buhagiar2, Adrian Mizzi1
Patient73 years, female
A 73-year-old lady with longstanding pancreatic insufficiency presented with a year-long history of epigastric pain on mild exertion, associated with fatigue, lumbosacral pain and mild bilateral lower limb oedema. A soft tissue mass lesion was noted in the wall of the inferior vena cava on Computed Tomography (CT) Pancreas.
CT Pancreas showed a soft tissue density mass in the wall of the infra- and intrahepatic inferior vena cava (IVC). The mass protruded into the lumen of the IVC and measured 5.3 x 3.1 x 3.9cm (Figure 1, 2). In retrospect, a filling defect could be seen in intrahepatic IVC on CT Pancreas done two years previously.
Magnetic Resonance (MR) imaging of the liver confirmed presence of soft tissue mass in wall and lumen of IVC with no additional findings (Figure 3-5).
PET/CT scan done utilising 18F-Fludeoxyglucose showed moderate increase in glucose metabolism limited to the IVC mass, with a mean standardised uptake value (SUV) of 4.4 (Figure 6).
Mass was biopsied under CT guidance through the posterior chest wall utilising an 18-gauge coaxial biopsy needle, with a single core sent for histology (Figure 7).
Leiomyosarcoma (LMS) is one of the most common types of soft tissue sarcomas. In contrast, primary LMS originating from the IVC is a rare malignant tumour arising from venous intimal smooth muscle [1]. It represents close to 0.5% of adult soft tissue sarcomas with a predilection for females (3:1 ratio) [2-4].
Classification is based on three segments which denote the relationship of the LMS to the IVC [5].
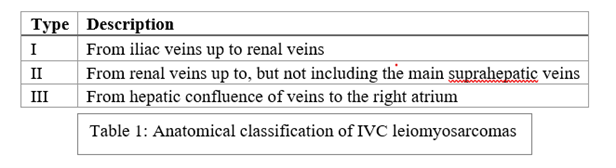
IVC leiomyosarcomas are associated with three tumour growth patterns: extraluminal (62%), intraluminal (5%), or a combination of both (33%) [4]. These tumours have a poor prognosis, although segment II tumours are associated with better outcomes [6], likely due to earlier presentation [7]. Most patients present with non-specific symptoms and the tumour is often an incidental finding. Nonetheless, there is some geographic variation; segment II lesions can present with nephrotic syndrome and right upper quadrant pain, due to involvement of the renal veins [4]. Infra-renal LMS tend to present with ascites and lower limb oedema, whereas segment III may present with Budd-Chiari syndrome and pulmonary vein thrombosis [8].
Negative prognostic features include tumour grade, the presence of both extraluminal and intraluminal growth - which was present in our patient’s case, and disseminated malignancy [9].
CT plays a crucial role in early and accurate diagnosis of LMS. MRI excels in showing invasion of adjacent anatomical structures and tumour thrombosis [10] resulting in a higher ratio of surgical resections and improvement of survival [11].
There are no pathognomonic radiological findings associated with LMS [11], with typical CT imaging findings varying depending on the tumour site. Extraluminal LMS typically appears as large, often more than 10cm, lobulated retroperitoneal mass, with no specific location predisposition and with varying attenuation, depending on presence of necrotic foci. Intravascular LMS generally manifest as a heterogeneous, intermediate-attenuation, slow-growing tumour mass arising along the anatomical path of the IVC, with IVC expansion and an indiscernible caval lumen [10]. They may be associated with development of an extensive significant collateral circulation [12]. Without use of contrast, an intraluminal LMS is extremely difficult to detect on CT [4]. Even in presence of IV contrast, endoluminal LMS can be easily missed on CT of the abdomen in portal venous phase, given that the IVC above the kidneys often manifests heterogeneous density due to mixing of enhanced blood from renal veins mixing with relatively unenhanced blood from lower limbs and pelvis.
En bloc surgical resection of the tumour, ideally with clear margins, remains the gold standard in management. Given its exceeding rarity, there is a paucity of clinical data with respect to use and benefit of adjuvant chemotherapy. Post-operative radiotherapy is also limited in benefit given the intrinsic radio-resistance of sarcomas and normal tissue tolerance to radiotherapy of surrounding vital organs. If the patient is precluded from surgical intervention because of anaesthetic concerns or tumour extent, then radiotherapy is used to slow down the rate of tumour growth.
Unfortunately, prognosis is poor with local recurrence in half of the patients, and 33% 5-year overall survival. Metastatic disease can be palliated with a variety of agents including anthracyclines-based chemotherapy or targeted therapy (such as tyrosine kinase inhibitors), as well as surgical excision or radiotherapy when appropriate.
Written informed patient consent for publication has been obtained.
[1] Mastoraki A, Leotsakos G, Mastoraki S, Papanikolaou IS, Danias N, Smyrniotis V (2015) Challenging diagnostic and therapeutic modalities for leiomyosarcoma of inferior vena cava. Int J Surg 13:92–5 (PMID: 25489949).
[2] Teixeira FJR, Netto SD do C, Perina AL de F, Torricelli FCM, Teixeira LR, Zerati AE (2017) Leiomyosarcoma of the inferior vena cava: Survival rate following radical resection. Oncol Lett 14(4):3909 (PMID: 29098019).
[3] Joung HS, Nooromid MJ, Eskandari MK, Wayne JD (2020) Surgical approach, management, and oncologic outcomes of primary leiomyosarcoma of the inferior vena cava: An institutional case series. J Surg Oncol 122(7):1348–55 (PMID: 32772373).
[4] Hartman DS, Hayes WS, Choyke PL, Tibbetts GP (1992) From the archives of the AFIP. Leiomyosarcoma of the retroperitoneum and inferior vena cava: radiologic-pathologic correlation. 12(6):1203–20 (PMID: 1439022).
[5] Kulaylat MN, Karakousis CP, Doerr RJ, Karamanoukian HL, O’Brien J, Peer R (1997) Leiomyosarcoma of the inferior vena cava: a clinicopathologic review and report of three cases. J Surg Oncol 65(3):205–17 (PMID: 9236931).
[6] Mingoli A, Cavallaro A, Sapienza P, Di Marzo L, Feldhaus RJ, Cavallari N (1996) International registry of inferior vena cava leiomyosarcoma: analysis of a world series on 218 patients. Anticancer Res 16(5B):3201–5 (PMID: 8920790).
[7] Wachtel H, Gupta M, Bartlett EK, Jackson BM, Kelz RR, Karakousis GC (2015) Outcomes after resection of leiomyosarcomas of the inferior vena cava: a pooled data analysis of 377 cases. Surg Oncol 24(1):21–7 (PMID: 25433957).
[8] Kieffer E, Alaoui M, Piette JC, Cacoub P, Chiche L (2006) Leiomyosarcoma of the inferior vena cava: experience in 22 cases. Ann Surg 244(2):289–95 (PMID: 16858193).
[9] Laskin WB, Fanburg-Smith JC, Burke AP, Kraszewska E, Fetsch JF, Miettinen M (2010) Leiomyosarcoma of the inferior vena cava: clinicopathologic study of 40 cases. Am J Surg Pathol 34(6):873–81 (PMID: 20463568).
[10] Cortecero JM, Dolores M, Rubio G, Romá AP (2015) Leiomyosarcoma of the inferior vena cava: AIRP best cases in radiologic-pathologic correlation. Radiological Society of North America, Inc. 2015;35(2):616–20 (PMID: 25763742).
[11] Blum U, Wildanger G, Windfuhr M, Laubenberger J, Freudenberg N, Munzar T (1995) Preoperative CT and MR imaging of inferior vena cava leiomyosarcoma. Eur J Radiol 20(1):23–7 (PMID: 7556247).
[12] Wang MX, Menias CO, Elsherif SB, Segaran N, Ganeshan D (2021) Current update on IVC leiomyosarcoma. Vol. 46, Abdominal Radiology. Springer; 2021. p. 5284–96 (PMID: 34415408).
| URL: | https://www.eurorad.org/case/17733 |
| DOI: | 10.35100/eurorad/case.17733 |
| ISSN: | 1563-4086 |
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.