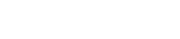
Initial non-contract CT head
Unchartered territory: Cerebral venous sinus and portal vein thrombosis post-COVID-19 vaccination
SectionNeuroradiology
Case TypeClinical Cases
Authors
Honida Mansour, Mustafa Hamza, Azhar Ali, Hadil Said, Harita Sivashankar, Katherine Harries, Sophia Maiguma-Wilson, Andrien Rajakumar, Rahul Sakarwadia, Noreen Rasheed, Sami Khan, Imran Syed
Patient22 years, male
A 22-year-old male with no previous medical history presented to hospital with right-sided hemiparesis preceded by a four-day history of headache and confusion. He received the ChAdOx1 nCoV-19 vaccination 10 days prior to admission. Blood investigations revealed marked thrombocytopenia (platelets 22 x109/L) and elevated D-Dimer (11624 ng/ml).
Initial non-contrast CT (NCCT) head revealed an expanded and hyperdense straight sinus (figure 1a) and superior sagittal sinus with a characteristic ‘delta sign’ (Figure 1b), suggestive of cerebral venous sinus thrombosis (CVST). CT cerebral venogram demonstrated an ‘empty delta sign’ with contrast outlining the triangular filling defect within the superior sagittal sinus and right transverse sinus; thereby confirming extensive CVST (Figure 2).
Following specialist haematological recommendation, immediate management of CVST included anticoagulation with Fondaparinux. One hour later, the patient developed new-onset seizures requiring sedation and intubation. Subsequent NCCT head revealed new bilateral cortical hyper-densities consistent with a subarachnoid haemorrhage (Figure 3).
Subsequent CT pulmonary angiogram, abdomen and pelvis, performed to screen for additional sites of thrombosis, revealed a right lower lobe segmental pulmonary embolism with further thrombosis of the left portal and anterior branch of the right portal vein.
Background
ChAdOx1 nCoV-19 vaccination related thrombogenic and thrombocytopenic reports amounted to 348 by late May 2021 on a background of 24.3 million first doses administered [1]. Thrombotic events typically occur 5-24 days post first immunisation. Thrombocytopenia, elevated D-Dimer levels, and positive antibodies to platelet factor 4 (PF4) have been identified and this rare complication has been named vaccine-induced immune thrombotic thrombocytopenia (VITT). Mortality from VITT is approximately 40% [2].
Clinical Perspective
Clinicians should maintain a high index of suspicion for CVST in young adults (mean age 33) presenting with headache and focal neurological deficits [3]. Although rare, a history of recent immunisation with COVID-19 adenovirus vector vaccines should raise diagnostic suspicion. CVST is potentially reversible with prompt diagnosis and anticoagulation. However, treatment carries a risk of bleeding, further complicated by existing thrombocytopenia seen in VITT [4]. A low threshold for repeat imaging to monitor disease progression and complications of treatment may be indicated.
Imaging Perspective
MRI venography remains the gold standard for diagnosis of CVST [5]. However, NCCT head is the most frequently used modality in the acute assessment of patients presenting with new-onset headaches, seizures, and focal neurological deficit. Evidence of CVST is suggested by presence of a hyperattenuated thrombus within an occluded sinus, referred to as ‘dense sinus sign’ [6]. Studies have shown a pooled sensitivity and specificity of NCCT in the detection of CVST to be 81% and 89% respectively. NCCT may at times only demonstrate indirect signs of CVST, namely diffuse brain oedema, mass effect and parenchymal haemorrhage [7].
CT venography utilises contrast and acquisition delay to provide a detailed depiction of the cerebral venous system. Studies have demonstrated 100% sensitivity and specificity. Following the administration of contrast, a thrombus within the superior sagittal sinus can be visualised as a filling defect, referred to as ‘empty delta sign’ [7]. Additionally, indirect signs of CVST demonstrated by NCCT can also be visualised [6].
Outcome
Following confirmation of diagnostic criteria of VITT, including positive PF4 antibodies [8], the patient was treated with intravenous immunoglobulins, therapeutic plasma exchange, and anticoagulation. Full recovery was observed one-month post-discharge.
Learning Points
To raise awareness of rare immune-mediated thrombotic events associated with COVID-19 immunisation, specifically CVST due to its high risk of morbidity and mortality. We highlight the importance of prompt and appropriate imaging, including additional imaging of the chest and abdomen in asymptomatic patients to investigate for further sites of thrombosis.
Written informed patient consent for publication has been obtained.
[1] Public Health England (2021) Blood clotting following COVID-19 vaccination information for health professionals. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/992072/PHE_COVID-19_AZ_vaccine_and_blood_clots_factsheet_8June21.pdf. Accessed (01/06/21)
[2] Lee A, Lee EJ (2021) Cerebral venous sinus thrombosis after vaccination: the UK experience. The Lancet S0140-6737: 1-2 (3437097)
[3] Ulivi L, Squitieri M, Cohen H et al (2020) Cerebral venous sinus thrombosis: a practical guide. BMJ 20: 1-12 (3295859)
[4] Furie KL, Cushman M, Elkind MS et al (2021) Diagnosis and management of cerebral venous sinus thrombosis with vaccine induced immune thrombotic thrombocytopenia. Stroke 52: 2478-2482 (33914590)
[5] Idiculla PS, Gurala D, Palanisamy M et al (2020) Cerebral venous sinus thrombosis: a comprehensive review. European Neurology 83: 369-379 (32877892)
[6] Avsenik J, Oblak JP, Popovic KS (2016) Non-contrast computed tomography in the diagnosis of cerebral venous thrombosis. Radiology and Oncology 50: 263-268 (27679541)
[7] Dam LF, Walderveen MA, Kroft LJ et al (2020) Current imaging modalities for diagnosing cerebral vein thrombosis- a critical review. Thrombosis research 189: 132-139 (32220779)
[8] Public Health England, Expert Haematology Panel (2021). Guidance from the Expert Haematology Panel (EHP) on Covid-19 Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT). https://b-s-h.org.uk/media/20075/guidance-version-22-20210903.pdf (accessed 01/09/21)
| URL: | https://www.eurorad.org/case/17515 |
| DOI: | 10.35100/eurorad/case.17515 |
| ISSN: | 1563-4086 |
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.